Magnetism
All materials consist of atoms and molecules which may or may not have a permanent magnetic influence depending on the electron configuration within the material. Atoms have positive nuclei and negative electrons.
Atoms in magnetic materials group together in regions called magnetic domains; each domain has its own north and south pole. When these domains are randomly positioned, the material is un-magnetised. If the domains are aligned in a common direction, then the material will be magnetised and the material itself will have its own north and south pole. Atoms have magnetic polarity parallel with the crystal axis, but the domains are randomly orientated. A typically sized domain is approximately 10²⁰ atoms.
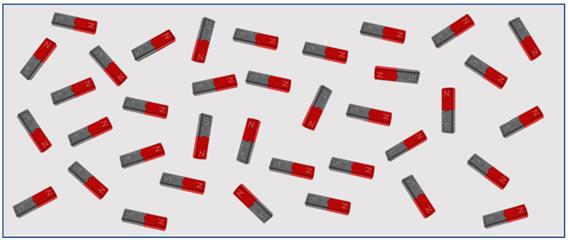
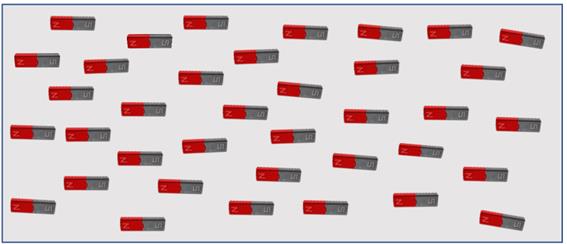
The domains can be aligned by bringing them within an existing magnetic field. If the domains remain aligned when they are removed from the influence of the magnetic field, then the material is said to be permanently magnetised. Magnetic material (quantum mechanical theory) depends upon unique atomic properties of spacing of the atoms in the crystalline lattice.
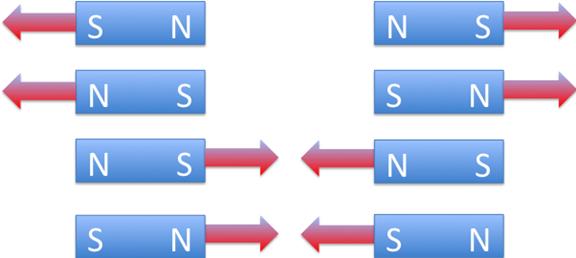
The poles of magnetised materials have an inherent attraction/ repulsion effect. A simpler definition would be ‘the properties of certain metals to attract or repel certain other metals.’ If two pieces of magnetised material are placed with their dissimilar poles end to end there is an attraction, but if the poles are alike then there is a repulsion, therefore: